Laura M. Poller
Graduate Student
ETH Zürich
Talk Information
Strategies for Membrane Permeability or Oral Bioavailability
16 June 2025, 09:10am - 09:20am, in the Pacific Jewel Ballroom
L04 - YI1 – Collagen Cross-Linking: Structure and Sequence Selectivity of Lysyl Oxidase-Like 2

Laura Marie Poller is a Ph.D. candidate in the Wennemers Group at ETH Zürich, where she explores the intersection of peptide chemistry and chemical biology. Her research focuses on developing innovative chemical tools to investigate enzyme activity and design functional biomaterials.
Academic Background
Ms. Poller is pursuing her doctoral studies under the mentorship of Professor Helma Wennemers in the Laboratory of Organic Chemistry at ETH Zürich. She holds a Kekulé Fellowship from the “Stiftung Stipendien-Fonds des Verbandes der Chemischen Industrie,” supporting her research endeavors. Her academic journey has been marked by a commitment to advancing the field of chemical biology through interdisciplinary approaches.
Research Focus
Poller's research centers on the design and synthesis of peptide-based probes to study enzymatic processes, particularly those involving lysyl oxidase. By creating turn-on fluorescent probes, she aims to enable real-time monitoring of enzyme activity, which has significant implications for understanding disease mechanisms and developing therapeutic strategies. Her work exemplifies the integration of synthetic chemistry with biological applications.
Notable Contributions
In recognition of her contributions to chemical biology, Poller received the Best Poster Presentation Award at the Swiss Summer School on Chemical Biology in 2024 for her work titled “Evaluating Lysyl Oxidase Activity with Turn-On Fluorescent Probes.” Her research has also been featured in high-impact journals, including a co-authored publication in Nature Communications on enhancing the cellular uptake of therapeutic oligonucleotides.
Professional Engagements
Beyond her research, Poller actively participates in the scientific community, presenting her findings at conferences and contributing to collaborative projects. Her dedication to advancing chemical biology and her innovative approach to research have established her as a promising young investigator in the field.
Through her interdisciplinary research and commitment to scientific excellence, Laura Marie Poller continues to make significant strides in chemical biology, exemplifying the impact of emerging scientists in advancing our understanding of complex biological systems.
Collagen Cross-Linking: Structure and Sequence Selectivity of Lysyl Oxidase-Like 2
Laboratory of Organic Chemistry, ETH Zurich, Vladimir-Prelog-Weg 3, 8093, Zurich, Switzerland
The remodelling and maturation of collagen, the dominant structural protein in mammals, is crucial for the integrity of organs and wound healing.1,2 A key process is the cross-linking of collagen strands triggered by the post-translational oxidative deamination of lysine to allysine residues through lysyl oxidases (LOXs).
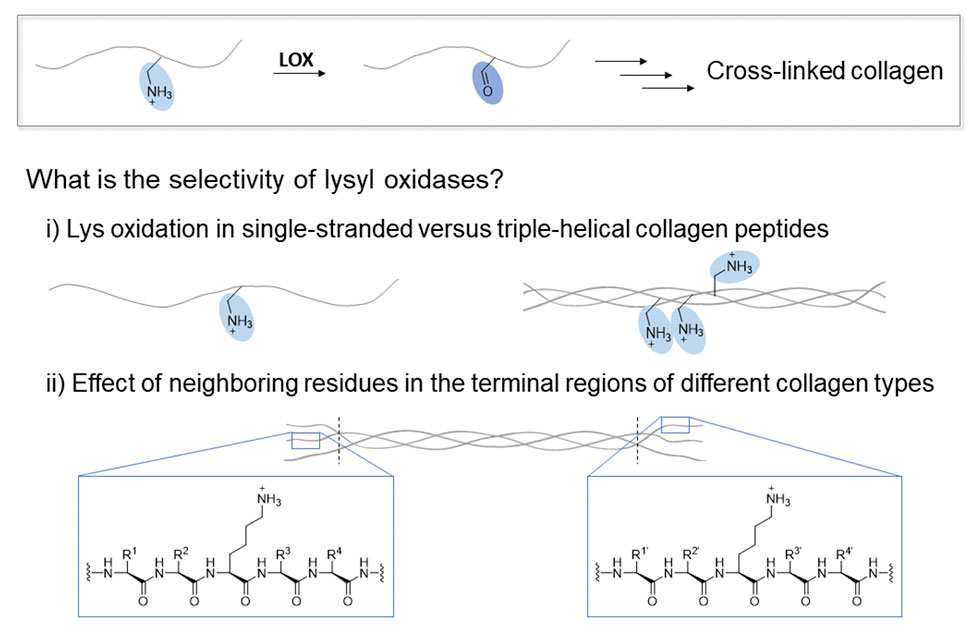
Excessive LOX activity is, however, implicated in fibrotic and malignant diseases which are associated with more than one third of deaths woldwide.1–3 Understanding the role of LOXs in specific tissues could facilitate addressing these diseases. Surprisingly little is, however, known about the selectivity of LOXs.
In this study, we deciphered structure and sequence effects on aldehyde formation by LOXL2. This LOX isoform is of particular interest as it is over-expressed in many cancers.4 Using fluorogenic enzyme activity assays, we investigated proline-rich peptides, comprising a lysine residue, that mimic either an intact or disrupted triple-helical structure, as found in connective tissue disorders.5
Our findings revealed the importance of the lysine-containing peptide sequence with a remarkable impact of charged residues on LOXL2 activity. We also show that LOXL2 is most active on single-stranded proline-rich peptides, as present in pathologically disturbed collagen triple helices.6

