Andy Wilson
Professor
University of Birmingham
Talk Information
Peptides in Oncology
18 June 2025, 10:55am - 11:10am, in the Pacific Jewel Ballroom
L41 – Understanding and Manipulating Protein-Protein Interactions of Aurora-A Kinase Employing Intrinsically Disordered Regions

Professor Andy J. Wilson serves as a Professor of Chemical Biology in the School of Chemistry at the University of Birmingham. His research focuses on the application of synthetic molecules to challenges in chemical biology and materials science, particularly in understanding and manipulating protein-protein interactions, PPIs relevant to health and disease.
Academic Background
Professor Wilson earned his B.Sc., Hons, in Chemistry from the University of Manchester Institute of Science and Technology, UMIST, in 1997. He completed his Ph.D. in Synthetic Chemistry under the supervision of Professor David A. Leigh FRS, focusing on the controlled assembly of interlocked architectures. Prior to his current position, he held academic appointments at the University of Leeds, where he led a research group specializing in supramolecular chemical biology and materials chemistry. In 2023, Professor Wilson and his research team joined the University of Birmingham, bringing their expertise to the institution's expanding initiatives in molecular sciences.
Research Focus
Professor Wilson's laboratory is dedicated to developing synthetic molecules that modulate PPIs, aiming to advance drug discovery and understand biological processes. His team employs chemical biology approaches to design inhibitors of PPIs, investigate amyloid assembly mechanisms, and develop chemical protein labeling techniques. Their work addresses challenges in targeting PPIs, which are often considered "undruggable," by creating molecules that can selectively and effectively disrupt these interactions.
Notable Contributions
Professor Wilson has significantly contributed to the field of chemical biology through the development of proteomimetic inhibitors that target intracellular PPIs. His research includes the design of constrained peptides and small molecules that mimic protein secondary structures, enabling the modulation of PPIs involved in diseases such as cancer and neurodegeneration. His work has been published in leading scientific journals, reflecting the impact of his research on understanding and controlling complex biological systems.
Professional Engagements
Beyond his research, Professor Wilson is actively involved in collaborative projects and interdisciplinary programs. He has led and participated in large-scale initiatives such as the EPSRC-funded PoPPI Programme and the BBSRC-supported SPiDR project, focusing on developing tools to study and manipulate PPIs. His leadership in these programs underscores his commitment to advancing the field through collaborative efforts that bridge chemistry and biology.
Through his innovative research and dedication to interdisciplinary collaboration, Professor Andy J. Wilson continues to make significant contributions to the understanding and manipulation of protein-protein interactions, with implications for therapeutic development and materials science.
Understanding and Manipulating Protein-Protein Interactions of Aurora-A Kinase Employing Intrinsically Disordered Regions
School of Chemistry, University of Birmingham, Edgbaston Campus, Birmingham, B15 2TT, United Kingdom
Intrinsically disordered regions (IDRs), are ubiquitous stretches of protein that do not adopt a stable structure, and a major class of protein structure found in all living organisms and viruses,1 The contribution of IDRs to spatiotemporal control of cellular protein interactions and function remains poorly understood. This presentation will discuss our efforts to explore IDRs in regulating the cellular functions of a common factor, the protein kinase Aurora-A, an essential mitotic Ser/Thr kinase needed for cell-cycle progression, which, has received attention, but to date proven challenging, as an anticancer target.2
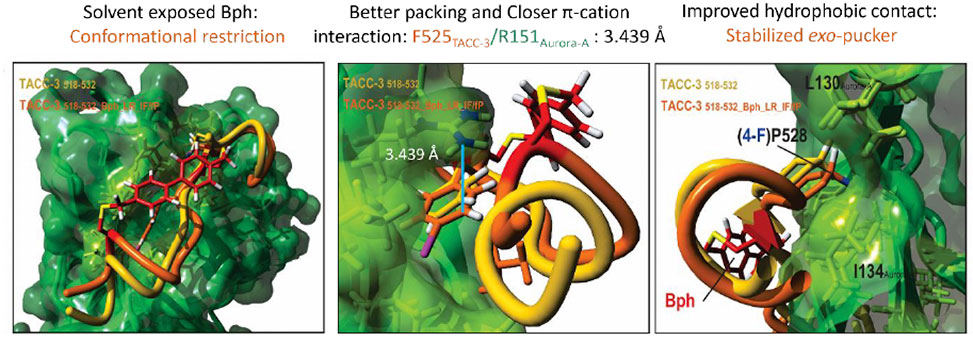
Figure 1. Aurora A binding TACC3 peptidomimetics: potent binding arises through a optimizing conformation effects and non-covalent contacts
Aurora-A is an incomplete kinase; its localization and activation are regulated through interaction with intrinsically disordered clients.3 This presentation will discuss the structural and functional characterization of new Aurora-A PPIs,4 alongside development of TACC3 peptidomimetics, Figure 15, above, that bind to Aurora-A and inhibit its interaction with TACC3 without inhibiting its enzymatic function. Excitingly, these peptidomimetics also allosterically inhibit the N-Myc/Aurora-A interaction opening new opportunities for drug discovery.
1 A. K. Dunker, et al., Intrinsically Disord. Proteins, 2013, 1, e24157.
2 J. Tischer, et al., J. Cell. Biol., 2018, 218, 10.
3 S. G. Burgess, et al., EMBO J., 2018, 37, e97902.
4 J. Holder, et al., EMBO J., 2024, 43, 5381.
5 D. Gimenez, et al., Chem. Sci., 2025, 16, 354.

