Chi Ting
Assistant Professor
Brandeis University
Talk Information
Implications and Applications of PTMs
19 June 2025, 02:35pm - 02:45pm, in the Pacific Jewel Ballroom
L67 – Chemical Synthesis of Ribosomally Synthesized and Post-translationally Modified Peptides (RiPPs)

Assistant Professor Chi P. Ting serves in the Department of Chemistry at Brandeis University. His research focuses on the total synthesis of natural products, chemoselective functionalization of peptides, and genome mining for natural product biosynthesis. His work aims to develop efficient synthetic methods and discover novel bioactive compounds.
Academic Background
Dr. Ting earned his B.S. in Chemistry from the University of Illinois at Urbana-Champaign in 2012, where he conducted undergraduate research under Professor Steven Zimmerman. He completed his Ph.D. in Chemistry at the University of California, Berkeley, in 2017, working with Professor Tom Maimone on the total synthesis of complex natural products. Following his doctoral studies, he returned to the University of Illinois for postdoctoral research in biochemistry with Professor Wilfred van der Donk, focusing on the biosynthesis of amino acid-derived natural products. In 2020, Dr. Ting joined Brandeis University as an Assistant Professor of Chemistry.
Research Focus
Professor Ting's laboratory integrates organic synthesis and biosynthesis to explore natural products. His team develops concise synthetic routes and new reactions to access motifs found in natural products, particularly focusing on chemoselective functionalization of peptides to study ribosomally synthesized and post-translationally modified peptides, RiPPs. Additionally, the lab employs genome mining techniques to identify biosynthetic gene clusters in microorganisms, aiming to discover new natural products and understand their biosynthetic pathways.
Notable Contributions
Dr. Ting has made significant contributions to the field of natural product synthesis and biosynthesis. His work includes the development of methods for the total synthesis of complex meroterpenes and the elucidation of biosynthetic pathways for amino acid-derived natural products. His research has been recognized with several awards, including the NSF CAREER Award and the Thieme Chemistry Award in 2024, as well as fellowships from the NIH and Bristol-Myers Squibb.
Professional Engagements
Beyond his research, Professor Ting is actively involved in mentoring students and contributing to the academic community at Brandeis University. He leads the Ting Research Group, fostering an environment that encourages interdisciplinary collaboration and innovation in chemical biology.
Through his innovative research and dedication to education, Assistant Professor Chi P. Ting continues to make significant contributions to the fields of organic synthesis and natural product biosynthesis.
Stereoselective Synthesis and Structural Assignment of Enteropeptin A
Brandeis University
Ribosomally synthesized and post-translationally modified peptides (RiPPs) are a growing class of natural products, many of which possess antimicrobial activity. Sactipeptides are one subclass of RiPPs that are defined by thioaminoketal functional groups. In this presentation, thioaminoketals are assembled through the Markovnikov hydrothiolation of dehydroamino acids using a dithiophosphoric acid catalyst. This method results in the formation of the thioaminoketal directly from peptides containing dehydroamino acids and overrides the inherent reactivity of thiols to undergo conjugate addition.
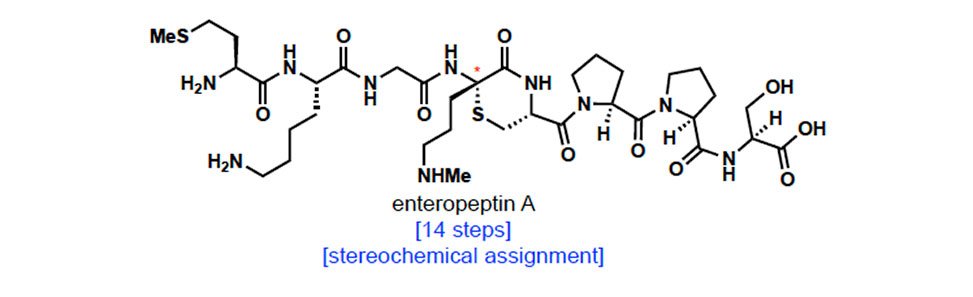
Despite their therapeutic potential, many sactipeptides are stereochemically undefined, preventing their further development into antibiotics. Enteropeptin A is an antimicrobial sactipeptide with a highly unusual thioaminoketal embedded in a thiomorpholine ring. The stereochemical configuration at its thioaminoketal stereocenter was not assigned when the natural product was isolated. In this presentation, the total synthesis of enteropeptin A is discussed. The synthesis of enteropeptin A and its diastereomer enabled the structural elucidation and the determination of the stereochemical configuration of enteropeptin A. In the synthesis, the Markovnikov hydrothiolation reaction was applied in a stereoselective cyclization to form the thiomorpholine ring. These results form a foundation and potential guidelines for the development of stereoselective peptide cyclization.

