Zhenquan Sun
Postdoctoral Scholar
University of Chicago
Talk Information
Peptide Synthesis and Innovation
16 June 2025, 02:50pm - 03:00pm, in the Pacific Jewel Ballroom
L14-YI3 – Chemical Synthesis of Difficult Peptide and Protein by N,O/S-Benzylidene (thio)Acetals (NBA) Strategy

Dr. Zhenquan Sun is a postdoctoral scholar in the Department of Medicine at the University of Chicago, where he specializes in chemical peptide and protein synthesis. His research focuses on developing innovative strategies for synthesizing and modifying complex biomolecules, with the aim of advancing chemical biology and therapeutic development.
Academic Background
Dr. Sun earned his Ph.D. in Chemical Biology from The University of Hong Kong in 2022. During his doctoral studies, he achieved the first total synthesis and structural elucidation of the calcium-dependent antibiotic Malacidin A by pioneering a β‑hydroxyaspartic acid ligation method that overcomes challenging peptide cyclization. He also holds a B.S. in Chemistry from Sun Yat-sen University. In addition to his academic pursuits, Dr. Sun has served as Chief Technology Officer at Serilink Biotechnology Company Limited, where he investigated next-generation bioconjugation methods to produce antibody-drug conjugates for novel therapeutic modalities.
Research Focus
Dr. Sun's research centers on addressing longstanding challenges in protein synthesis. He developed the N,O/S-Benzylidene (thio)Acetal, NBA, strategy to tackle aggregation issues in protein synthesis, facilitating the production of difficult substrates such as chemokines, hormones, glycosylated proteins, and the native immune checkpoint protein PD-L1 IgV domain. Additionally, he pioneered a superfast organoboron-based desulfurization chemistry to streamline polypeptide assembly, demonstrated with the LAIR-1 cytoplasmic domain and serotonylated Histone. These methods open new avenues for accessing challenging peptides and proteins for fundamental research and therapeutic development.
Notable Contributions
Dr. Sun's innovative methodologies have been recognized for their impact on the field of chemical biology. His work on the total synthesis of Malacidin A and the development of the NBA strategy has provided valuable tools for the synthesis of complex peptides and proteins. His research has been featured in peer-reviewed publications, contributing to the advancement of peptide-based therapeutics and protein engineering.
Professional Engagements
Beyond his research, Dr. Sun actively engages with the scientific community through seminars and collaborations. He has presented his work at various conferences, including the BSD Postdoctoral Affairs seminar series at the University of Chicago. His commitment to advancing chemical biology is evident in his collaborative efforts and contributions to the development of novel therapeutic strategies.
Through his innovative research and dedication to the field, Dr. Zhenquan Sun continues to make significant contributions to chemical biology and the development of peptide-based therapeutics.
Chemical Synthesis of Difficult Peptide and Protein by N,O/S-Benzylidene (thio)Acetals (NBA) Strategy
a Department of Chemistry, University of Hong Kong, Hong Kong SAR
b Department of Medicine, University of Chicago, Chicago, IL, 60637, USA (Current address)
The development of solid phase peptide synthesis (SPPS) and chemical ligation strategies enable the chemical synthesis of peptide or protein at atomic level precision.1 However, difficult peptide/protein that are highly prone to aggregate, are still challenging to be synthesized due to their serious residue deletion in SPPS, poor reactivity in ligation and miserable separation in purification. Herein we present a novel and general strategy based on the N,O/S-benzylidene (thio)acetal (NBA) to address above obstacles.
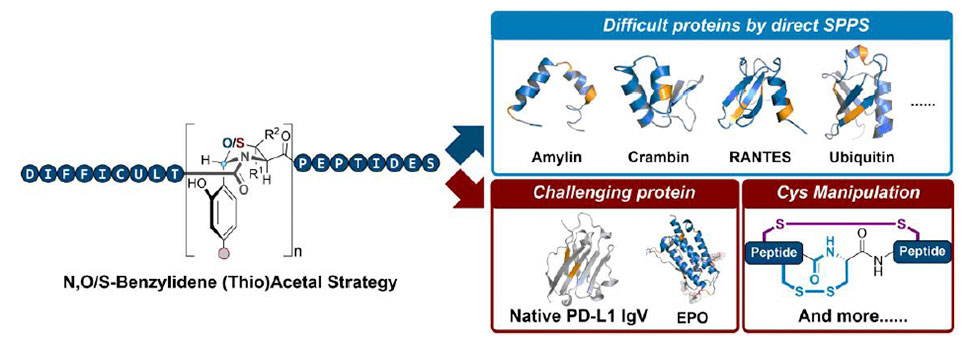
During the peptide synthesis, the twisted NBA structure from the Ser/Thr ligation serves as an efficient aggregation disruptor. Also, both O- and S- NBA scaffolds can be readily introduced at different stages of synthesis, either by the SPPS coupling of easily prepared building blocks2, 5 or via late-stage chemical ligation3.
Meanwhile, the unnatural Cys/Pen residue in the S-NBA can be converted into Ala/Val by desulfurization,4 covering broader substrate scope of NBA insertion. The utility of NBA strategy was further demonstrated by successful syntheses of various challenging peptides and proteins, including amylin, crambin, RANTES, ubiquitin, histones, erythropoietin and human programmed death ligand 1 (PD-L1 IgV). To sum up, the NBA strategy provides a flexible and robust platform to synthesize difficult peptides and proteins in an efficient way.
[1] Sun, Z.; Liu, H.; Li, X. Chem, 2024, 10, 767–799.
[2] Wu, H.; Sun, Z.; Li, X. Angew. Chem. Int. Ed. 2023, e202310624.
[3] Wu, H.#; Sun, Z.#; Li, X. Angew. Chem. Int. Ed. 2024, e202403396 (# equal contribution).
[4] Sun, Z. et al. Chem, 2022, 8, 2543–2557.
[5] Sun, Z.#; Wu, H.#; Zhang, Y.#; Li, X. in preparation.

