Timothy Reichart
Elliott Assistant Professor of Chemistry
Hampden-Sydney College
Talk Information
Peptide Synthesis and Innovation
16 June 2025, 03:00pm - 03:15pm, in the Pacific Jewel Ballroom
L15 – Total Chemical Synthesis of a 97-Amino Acid Target-Binding Monobody via Cysteine-Free Conformationally-Assisted Ligation

Dr. Timothy M. Reichart serves as the Elliott Assistant Professor of Chemistry at Hampden-Sydney College. His research focuses on peptide and protein chemistry, particularly the study of transmembrane domains from viral proteins such as SARS-CoV-2 and HIV. He is also interested in the development of peptides as catalysts for organic reactions, the chemical synthesis of large proteins using self-assembly, and the discovery of new potential antibiotics.
Academic Background
Dr. Reichart earned his B.S. from the University of Virginia in 2007 and his Ph.D. from The Scripps Research Institute in 2014. He completed postdoctoral training at the U.S. Army Combat Capabilities Development Command Soldier Center and the University of Massachusetts Dartmouth from 2014 to 2016, followed by a fellowship at the École Polytechnique Fédérale de Lausanne in Switzerland from 2016 to 2020.
Research Focus
Dr. Reichart's research encompasses several areas of peptide and protein chemistry. One current focus is the study of transmembrane domains from viral proteins, including SARS-CoV-2 and HIV. Other research areas include the use of peptides as catalysts for organic reactions, the chemical synthesis of large proteins using self-assembly, and the discovery of new potential antibiotics.
Notable Contributions
Dr. Reichart has received several honors and awards, including the Marie Sklodowska-Curie Fellowship / EPFL Fellow Co-Fund, 2017–2019, the National Research Council of the National Academy of Sciences Research Associateship in Chemical and Biological Defense, 2014–2016, and the California HIV/AIDS Research Program Fellowship, 2011–2013.
Professional Engagements
At Hampden-Sydney College, Dr. Reichart teaches a range of chemistry courses, including Techniques of Chemistry, Intermediate Laboratory, Advanced Laboratory, and Biochemistry I and II. He is committed to mentoring students and involving them in research projects that explore various aspects of peptide and protein chemistry.
Through his innovative research and dedication to education, Dr. Timothy M. Reichart continues to make significant contributions to the field of chemistry and to the academic community at Hampden-Sydney College.
Total Chemical Synthesis of a 97-Amino Acid Target-Binding Monobody via Cysteine-Free Conformationally-Assisted Ligation
Hampden-Sydney College
Native chemical ligation enables stepwise assembly of proteins via unprotected ligation at cysteine.1 Total chemical synthesis of proteins is limited by the need to strategically place cysteines for native chemical ligation with possible subsequent desulfurization. In special cases where different protein fragments self-assemble, the resulting high effective concentration allows direct aminolysis of the thioester, enabling ligation without a cysteine.2
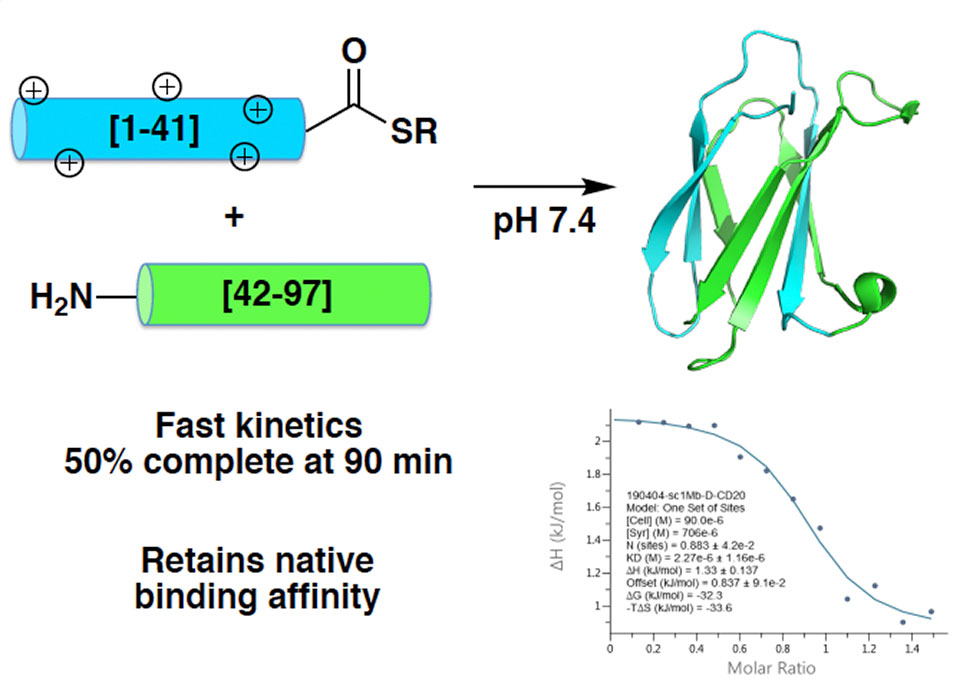
Figure 1. Conformationally-assisted ligation proof of concept using monobodies, synthetic binding proteins based on the tenth fibronectin III domain.
We have applied this principle to the synthesis of a 97-amino acid β-sheet monobody protein based on the tenth fibronectin type III domain. This required the combination of two separate strategies. First, the insoluble N-terminal portion was selectively modified by arginine substitution to enhance solubility.3 Second, the disconnection was chosen to permit self-assembly, maximizing high local concentration, and permitting rapid, direct aminolysis. The resulting protein retained binding to its original target as measured by ITC.
1. Dawson, P., Muir, T., Clark-Lewis, I., and Kent, S. Science 1994, 266, 776–779.
2. Beligere, G.S., and Dawson, P.E. J. Am. Chem. Soc. 1999, 121, 6332–6333.
3. [Reference for arginine substitution not provided in abstract.]

