James Nowick
Distinguished Professor
University of California, Irvine
Talk Information
Bioactive Peptides
19 June 2025, 10:40am - 11:05am, in the Pacific Jewel Ballroom
l58 – Potent Peptide Antibiotics Against Pernicious Pathogens

Distinguished Professor James S. Nowick is a faculty member in the Department of Chemistry at the University of California, Irvine. Renowned for his pioneering work in peptidomimetic chemistry and supramolecular assemblies, Professor Nowick's research has significantly advanced the understanding of amyloid diseases and antibiotic development.
Academic Background
Dr. Nowick earned his A.B. in Chemistry from Columbia University in 1985 and his Ph.D. in Organic Chemistry from the Massachusetts Institute of Technology in 1990, under the mentorship of Professor Rick L. Danheiser. Following an NSF postdoctoral fellowship with Professor Julius Rebek at MIT, he joined UC Irvine as an Assistant Professor in 1991, rising to full Professor in 1998. He served as Chair of the Department of Chemistry from 2016 to 2019 and holds a joint appointment in the Department of Pharmaceutical Sciences.
Research Focus
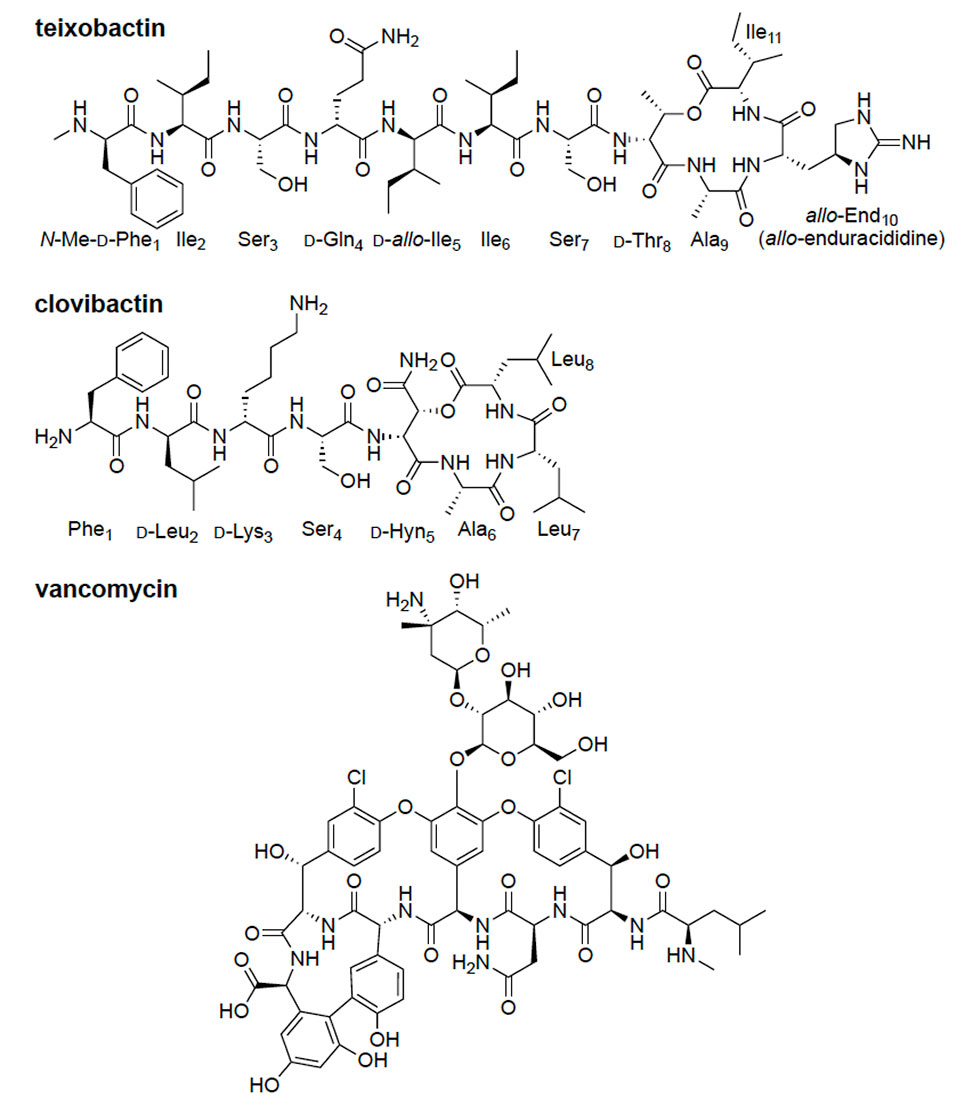
Professor Nowick's research centers on the design and synthesis of peptidomimetic molecules that replicate β-sheet structures, aiming to understand and disrupt protein-protein interactions involved in amyloid diseases such as Alzheimer's. His group also investigates the supramolecular chemistry of antibiotics like teixobactin, exploring how self-assembly contributes to their biological activity and developing analogues with improved properties.
Notable Contributions
Dr. Nowick has authored over 140 publications, with significant contributions to the understanding of β-sheet interactions and the development of artificial β-sheets. His work on teixobactin analogues has provided insights into antibiotic self-assembly and function. Additionally, he has been instrumental in promoting open-access education through the Open Chemistry initiative, offering free online chemistry lectures to a global audience.
Awards and Honors
Professor Nowick's achievements have been recognized with numerous awards, including:
- Arthur C. Cope Scholar Award, American Chemical Society, 1998
- Camille Dreyfus Teacher-Scholar Award, 1996
- Presidential Faculty Fellow Award, 1995
- Alfred P. Sloan Research Fellowship, 1997
- Fellow of the American Association for the Advancement of Science
- Fellow of the American Chemical Society
Professional Engagements
Beyond his research, Professor Nowick is dedicated to education and outreach. He has received multiple awards for excellence in undergraduate teaching and is actively involved in mentoring students. His commitment to diversity and inclusion is evident through his advocacy for LGBTQ+ representation in science and the establishment of supportive programs for underrepresented groups in chemistry.
Through his innovative research, educational initiatives, and advocacy, Professor James S. Nowick continues to make significant contributions to the fields of chemistry and chemical biology.
Potent Peptide Antibiotics Against Pernicious Pathogens
Department of Chemistry, University of California, Irvine, Irvine, California, USA
New antibiotics of last resort are desperately needed to treat antibiotic-resistant infections from pathogens such as MRSA and VRE. This talk will describe how my research group has overcome problems associated with teixobactin and clovibactin, two promising new peptide antibiotics against Gram-positive bacteria that have not yet made it into the clinic.
The talk will also describe how we have overcome vancomycin resistance through the creation of vancomycin–teixobactin conjugates. Each of these projects has yielded potent new antibiotics with improved pharmacological properties over the naturally occurring antibiotics.
It is hoped that these studies will not only illuminate promising preclinical drug candidates but also shed light on three fascinating peptide antibiotics that achieve their remarkable mechanisms of action through molecular recognition and supramolecular assembly.