Sikabwe Noki
Postdoctoral Research Fellow
University of KwaZulu-Natal
Talk Information
Sustainability in Peptide Science
17 June 2025, 09:10am - 09:20am, in the Pacific Jewel Ballroom
L25 - YI5 – "Base Labile" Safety Catch Linker. Synthesis and Applications in SPPS in a Green Context

Sikabwe Noki is a Postdoctoral Research Fellow in the School of Chemistry and Physics at the University of KwaZulu-Natal, UKZN. His research focuses on peptide chemistry, particularly the design and synthesis of peptide-based compounds with potential therapeutic applications.
Academic Background
Dr. Noki obtained his Bachelor of Science in Chemistry and a master's degree in Peptide Chemistry from the University of KwaZulu-Natal. He is currently completing his doctoral studies in Organic Chemistry, specializing in peptide chemistry, at the same institution. He is also a member of the Golden Key International Honor Society, recognizing his academic excellence.
Research Focus
Dr. Noki's research centers on the chemical synthesis of peptides and peptidomimetics, aiming to develop novel compounds for pharmaceutical applications. His work includes exploring safety-catch linkers and thiobarbituric acid derivatives in peptide synthesis, contributing to advancements in the field of medicinal chemistry.
Professional Engagements
Dr. Noki has been recognized as a Young Investigator Speaker at the upcoming American Peptide Symposium, highlighting his contributions to peptide science. He actively participates in scientific conferences and collaborates with researchers to further the understanding and application of peptide-based therapeutics.
Through his dedication to research and innovation in peptide chemistry, Dr. Sikabwe Noki continues to contribute to the development of novel therapeutic agents and the advancement of chemical sciences.
“Base Labile” Safety Catch Linker: Synthesis and Applications in SPPS in a Green Context
1 Peptide Science Laboratory, School of Chemistry and Physics, University of KwaZulu-Natal, Westville, Durban 4000, South Africa
2 School of Laboratory Medicine and Medical Sciences, College of Health Sciences, University of KwaZulu-Natal, Durban 4041, South Africa
3 AAPPTec, 6309 Shepherdsville Road, Louisville, Kentucky 40228, USA
Safety-catch linkers and protecting groups (PGs) play a significant role in peptide synthesis by allowing precise control of their stability: stable during the synthetic process in a broad range of chemical conditions and labile after chemical manipulation in one of the conditions that previously were stable. “Base-labile” safety-catch linkers and PGs offer a strategic advantage due to their stability under acidic, for example, TFA, and basic conditions, for example, piperidine, followed by selective cleavage under basic conditions, facilitating α-amino deprotection and final cleavage in solid phase peptides synthesis.
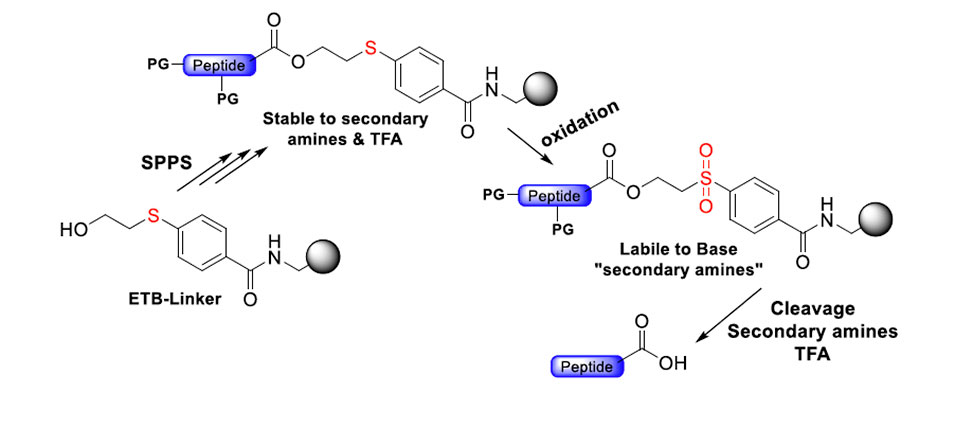
This study introduces a base labile safety catch linker (ETB Linker) based on a sulfinyl moiety, which allows peptide elongations using Fmoc chemistry. After the sulfinyl group is oxidated to sulfone, peptides are released via a β-elimination using a secondary amine, for example, diethylamine, piperidine. This ETB Linker was synthesized in a three-step and attached to aminomethyl resin to form ETB resin. Optimization of key reaction was achieved using a multi-detachable system, allowing precise control over linker and peptide release.
Traditional cleavage reagents like TFA, TFMSA, TFE pose sustainability challenges due to polyfluoroalkyl substance (PFAS) hazardous nature. ETB resin reduces dependence on these reagents, offering a greener alternative for the cleaving protected and unprotected peptides. The resin’s efficacy was demonstrated in synthesizing longer peptides, such as peptide SAK and a 16-mer protected peptide (N-terminal sequence of liraglutide), proving its utility as a free-acid method for protected peptide preparation. Additionally, the ETB-linker was explored for substituting TFA in removing sidechain protecting groups, aligning with green chemistry context.

