Thu Nguyen
Ph.D. Candidate in Chemistry
New York University
Talk Information
Protein Modification, Structural Insights, and Disease State
18 June 2025, 09:10am - 09:20am, in the Pacific Jewel Ballroom
L36 - YI7 – A Proteomimetic Strategy for Modulation of Intrinsically Disordered Protein MYC

Thu Nguyen is a Ph.D. candidate in Chemistry at New York University, conducting her research in the Arora Lab. Her work focuses on the design and synthesis of peptide-based molecules to modulate protein-protein interactions, aiming to develop novel therapeutic strategies.
Academic Background
Thu earned her B.S. in Chemistry from the University of California, Irvine, where she engaged in research on directed evolution of proteases under Professor Greg Weiss. In 2019, she commenced her doctoral studies at NYU, joining the Arora Lab to further explore chemical biology and peptide chemistry.
Research Focus
In the Arora Lab, Thu's research centers on developing synthetic peptides and peptidomimetics to disrupt aberrant protein-protein interactions implicated in diseases such as cancer. Her work involves the design of helix mimetics and other constrained peptides to achieve high specificity and affinity for target proteins.
Professional Engagements
Beyond her research, Thu is actively involved in the scientific community, participating in conferences and collaborative projects. She is committed to advancing the field of chemical biology through innovative research and interdisciplinary collaboration.
Through her dedication to research and scientific inquiry, Thu Nguyen continues to contribute to the development of novel approaches in targeting protein-protein interactions for therapeutic applications.
A Proteomimetic Strategy for Modulation of Intrinsically Disordered Protein MYC
New York University
Traditional approaches to target proteins have relied on the paradigm that the unique three-dimensional folds of proteins provide ligandable binding sites. Conformationally dynamic proteins increase the level of difficulty in ligand design and the challenge is further exacerbated for proteins that are intrinsically disordered.
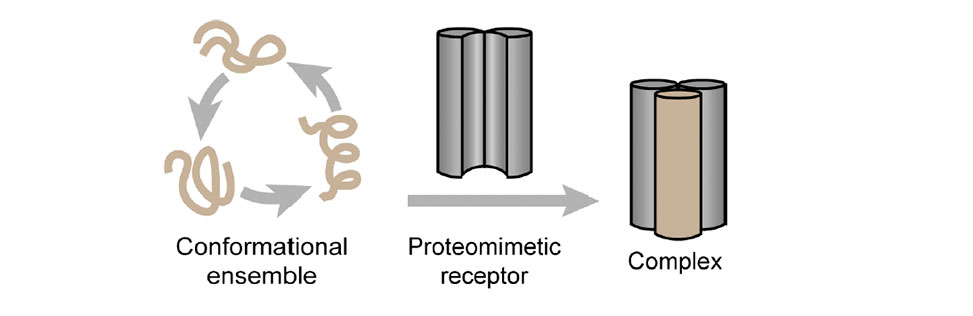
We hypothesized that one avenue for the development of binders for a disordered region would be to trap one of its thermodynamically accessible conformations with a receptor. Here we show the application of this approach to MYC, which represents a critical therapeutic target but has not yielded to small molecule inhibitors due to its conformationally dynamic nature. MYC adopts a helical configuration when it binds its cellular partner MAX.
We rationally designed a proteomimetic scaffold, termed Crosslinked Helix Dimers (CHDs), to trap this conformation. We show that MYC can be directly engaged both in biochemical and cellular assays. Overall, this work demonstrates a general method to capture and trap intrinsically disordered proteins with a propensity to adopt α-helical conformations.
‡ these authors contributed equally

