Mark Distefano
Distinguished McKnight University Professor
University of Minnesota
Talk Information
Implications and Applications of PTMs
19 June 2025, 02:10pm - 02:25pm, in the Pacific Jewel Ballroom
L65 – Synthetic Peptides and Modified Forms of Full-Length K-Ras4B, Prepared via Expressed Protein Ligation, Provide Insights into its Posttranslational Modification

Professor Mark D. Distefano is a distinguished chemist and educator at the University of Minnesota, holding the titles of Distinguished McKnight University Professor and Distinguished University Teaching Professor in the Department of Chemistry. His interdisciplinary work bridges organic chemistry and chemical biology, with a focus on protein engineering and enzymology.
Academic Background
Dr. Distefano earned his B.A. in Biochemistry and Chemistry from the University of California, Berkeley, in 1984. He completed his Ph.D. in Chemistry at the Massachusetts Institute of Technology in 1989, working under the mentorship of Professor Christopher T. Walsh. Following his doctoral studies, he conducted postdoctoral research in the laboratory of Professor Peter B. Dervan at the California Institute of Technology from 1989 to 1992.
Research Focus
Professor Distefano's research centers on the study of protein prenylation, a post-translational modification involving the attachment of isoprenoid groups to proteins, which plays a crucial role in cellular signaling. His lab develops chemical tools and methodologies to investigate the enzymes responsible for prenylation, aiming to understand their specificity and function. This work has implications for the development of therapeutic agents targeting diseases such as cancer, Alzheimer's, and infectious diseases. Additionally, his group explores the design of protein-based catalysts and the development of methods for selective protein modification.
Notable Contributions
Dr. Distefano has made significant contributions to the field of chemical biology through his innovative research on protein modifications. His work has advanced the understanding of enzyme mechanisms and has led to the development of novel biochemical tools. He has authored numerous peer-reviewed publications and holds several patents related to his research.
Awards and Honors
Professor Distefano's achievements have been recognized with several prestigious awards, including:
- American Chemical Society Fellow, 2014
- Distinguished McKnight University Professor, 2011
- Merck Professor of Chemistry, 2011
- Horace T. Morse-University of Minnesota Distinguished Teaching Professor, 2006
- George W. Taylor/ITAS Award for Distinguished Teaching, 2004
- National Science Foundation CAREER Award, 1994
- American Cancer Society Junior Faculty Research Award, 1994
Professional Engagements
Beyond his research, Dr. Distefano is actively involved in academic service and mentorship. He has served as Director of Graduate Studies in Chemistry and is a member of the University of Minnesota's Biotechnology Institute. His commitment to education is evident in his dedication to teaching and mentoring students at all levels.
Through his interdisciplinary approach and commitment to excellence, Professor Mark D. Distefano continues to make impactful contributions to the fields of chemistry and chemical biology.
Synthetic Peptides and Modified Forms of Full-Length K-Ras4B, Prepared via Expressed Protein Ligation, Provide Insights into Its Posttranslational Modification
Department of Chemistry, University of Minnesota, Minneapolis, USA; Department of Chemistry, Tsinghua University, Beijing, China
Mutations in K-Ras4B are drivers in approximately 20% of all human cancers1. The protein undergoes posttranslational modification steps including prenylation, proteolysis, and methylation near its C-terminus. To gain insight into this complex process, full-length forms of K-Ras4B containing various chemical probes have been prepared via expressed protein ligation between an N-terminal thioester (green) and a 15-residue C-terminal peptide (blue)2.
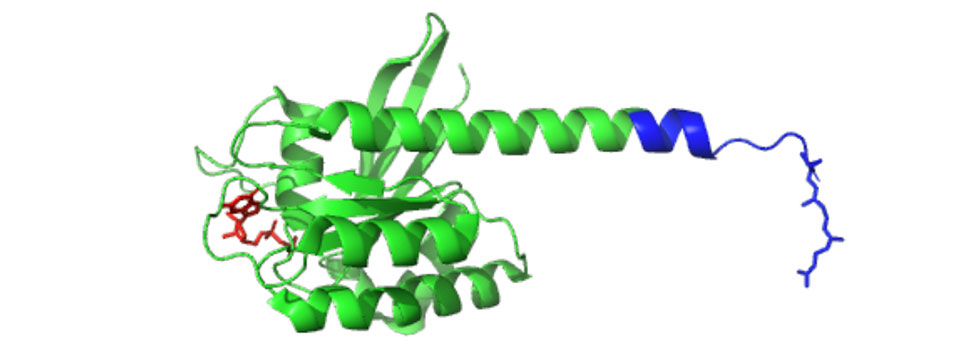
Synthetic peptides containing residues incorporating non-natural dansyl groups were used to study the rate of prenylation while peptides incorporating diazirine-containing isoprenoids allowed interacting proteins to be identified. Confocal imaging of fluorescently labeled K-Ras4B allowed its localization to be followed in living cells. Incorporation of peptides containing orthogonal azide- and alkyne-containing isoprenoids allowed the assembly of a covalent dimer.
1. Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ. Nat. Rev. Drug Discov. 2014, 13, 828–851.
2. Zhang SY, Sperlich S, Li FY, Al-Ayoubi S, Chen HX, Zhao YF, Li YM, Weise K, Winter R, Chen YX. ACS Chem. Biol. 2017, 12, 1703–1710.

